St. Luke's Health joins CommonSpirit.org soon! Enjoy a seamless, patient-centered digital experience. Learn more
Cancer (Oncology) — Dan L Duncan Comprehensive Cancer Center
Baylor St. Luke’s Medical Center is an internationally recognized leader in innovation, research and clinical excellence that has given rise to breakthroughs in cardiovascular care, neuroscience, oncology, transplantation, and more. Our team’s efforts have led to the creation of many research programs and initiatives to develop advanced treatments found nowhere else in the world.
Our strong alliance with Baylor College of Medicine allows us to bring our patients a powerful network of care unlike any other. Our collaboration is focused on increasing access to care through a growing network of leading specialists and revolutionizing healthcare to save lives and improve the health of the communities we serve.
Baylor St. Luke’s Medical Center is also the first hospital in Texas and the Southwest designated a Magnet® hospital for Nursing Excellence by the American Nurses Credentialing Center, receiving the award six consecutive times.
Baylor St. Luke’s Medical Center is the clinical home for the Dan L Duncan Comprehensive Cancer Center (DLDCCC) at Baylor College of Medicine, one of only three NCI-designated Comprehensive Cancer Centers in Texas.
The Dan L Duncan Comprehensive Cancer Center at Baylor St. Luke's Medical Center extends innovative cancer care to the Greater Houston area community. In collaboration with Baylor College of Medicine, the DLDCCC is equipped to pave the way for even more revolutionary discoveries and groundbreaking approaches to cancer prevention, diagnosis, and treatment. Our team is committed to providing a full continuum of care, high-touch patient support, and access to advanced resources.
Dan L Duncan Comprehensive Cancer Center at Baylor St. Luke’s Medical Center has launched the Albert and Margaret Alkek Foundation Center for Experimental Therapeutics, a Phase 1 clinical trial program that will provide its patients the opportunity to enroll in early phase clinical trials targeting a wide range of malignancies. The program’s first trial, which includes a novel therapy targeting a mutation commonly found in pancreatic and colorectal cancers, enrolled its first patient in August 2024.
Phase 1 trials are the first step in bringing innovative therapies that have shown promise in the laboratory to the clinic to demonstrate their safety and feasibility. The new program will build on the success of previous phase 1 trials at Baylor and provide robust infrastructure to offer more clinical trial opportunities to our patients.
Clinical trials in the Phase 1 program will investigate therapies developed by scientists within and outside of Baylor College of Medicine. Patients will receive treatment at the Dan L Duncan Comprehensive Cancer Center’s clinical home, Baylor St. Luke’s Medical Center’s state-of-the-art O’Quinn Medical Tower at the McNair Campus.
“Patients in these trials are often selected because their tumors have a molecular feature that is targeted by these therapies,” said Dr. S. Gail Eckhardt, who holds Baylor’s Albert and Margaret Alkek Endowed Chair and serves as associate dean for experimental therapeutics at Baylor and associate director of translational research at the Duncan Cancer Center.
The Phase 1 program’s team of clinical investigators consists of experts from all specialty areas, and Duncan Cancer Center patients will be able to continue care with their subspecialty provider while participating in a trial. Physicians from outside Baylor will also be able to refer patients to the Phase I program through an expedited and seamless referral process.
Oncology researchers at Baylor College of Medicine's Dan L Duncan Comprehensive Cancer Center are building on a novel chemoimmunotherapeutic approach that shows promise for reducing tumors in people with advanced pancreatic ductal adenocarcinoma.
Pancreas cancer is the third leading cause of cancer-related deaths in the United States, after lung and colon cancer. Pancreatic ductal adenocarcinoma is the most common form of pancreatic cancer, making up more than 90% of cases. The disease begins in the cells of the pancreas’ ducts, which transport juices containing digestive enzymes into the small intestine, and is often not detected until it is in an advanced stage. Pancreatic cancer has the highest mortality rate of any major cancer. It is estimated that in 2024, more than 66,000 Americans were diagnosed with pancreatic cancer, and more than 51,750 died from it.
In a phase I/II clinical study, researchers aimed to evaluate the safety and feasibility of combining LOAd703, an oncolytic virus-based immunostimulatory gene therapy, with chemotherapy for treating unresectable or metastatic pancreas cancer. The disease is characterized by low immunogenicity and an immunosuppressive tumor microenvironment. LOAd703 lyses cancer cells selectively, activates cytotoxic T cells, and induces tumor regression in preclinical models.
Combining LOAd703 with nab-paclitaxel plus gemcitabine in patients with advanced pancreatic ductal adenocarcinoma was found to be feasible and safe. To build upon this novel chemoimmunotherapeutic approach, phase II of the study, which combines LOAd703, nab-paclitaxel plus gemcitabine, and atezolizumab, is ongoing.
Advances in non-statin therapy, both available and those still in development, are giving hope to patients at risk of cardiovascular diseases who cannot tolerate or have a poor response to traditional statin therapy.
For decades, statin therapy has been the leading treatment for the management of dyslipidemia and atherosclerotic cardiovascular disease (ASCVD) risks. However, some patients, as a result of statin intolerance or inadequate response to therapy, require additional means to achieve a more optimal lipid profile.
Recently, leading cardiologist and trialist Christie Ballantyne, MD, professor of Medicine and chief of Cardiology and Cardiovascular Research at Baylor College of Medicine, called attention to a number of underutilized breakthroughs in nonstatin therapy to occur during the last decade that are effective in treating hypercholesterolemia (HeFH) or established ASCVD who require additional lowering of LDL-C—often called "bad" cholesterol—that is a major contributor to plaque buildup in the arteries that can lead to heart disease and stroke.
These non-statin therapy breakthroughs, highlighted in a presentation Dr. Ballantyne delivered at the Family Heart Foundation’s 2024 Family Heart Global Summit, include PCSK9 inhibitors (alirocumab and evolocumab), bempedoic acid and inclisiran, which have received endorsements for use in the management of hypercholesterolemia by the American College of Cardiology and American Heart Association since 2018. Also included is ezetimibe, which earned approval from the US Food and Drug Administration (FDA) more than 20 years ago.
These non-statin drugs are an effective adjunct to diet in helping to lower LDL-C in patients (both those who can tolerate statin therapy and those who can’t) with elevated LDL-C who have an increased risk of cardiovascular disease.
A team of researchers at Baylor College of Medicine and collaborating institutions is studying graft-vs-host disease (GVHD), an inflammatory reaction that is a major cause of mortality after bone marrow transplantation, a potentially curative therapy for many blood diseases.
In GVHD, immune T cells from the bone marrow transplant donor attack the host gut cells, mainly intestinal stem cells (ISCs). Researchers investigated the consequences of inflammation on ISCs.
Using cellular and animal models, researchers found that exposure to inflammation drove ISCs to change their metabolism in ways that resulted in the accumulation of succinate, a product of cellular processes, which in turn reprogramed the epigenome. (The epigenome is a system of chemical marks on the DNA that regulates the genes expressed by the cell.)
Inflammation-led epigenome reprogramming changed the expression of genes involved in cell reproduction, which is a first step toward healing the intestine. Overall, reprogrammed ISCs were less able to regenerate.
Researchers then investigated whether ISCs would be able to recuperate their regenerative ability after inflammation had resolved and found that ISCs had not overcome their initial exposure to inflammation. Despite mitigating GVHD inflammation for 28 days, ISCs retained a reduced regenerative capacity. In animal models, this led to poor recovery and increased mortality from challenges such as non-lethal radiation exposure.
More research is on the way to design strategies to help ISCs “forget” their encounter with inflammation and enhance their resilience against immune attacks.
In the search for new treatments for hard-to-cure cancers, researchers at Baylor College of Medicine employed computer modeling to discover a new compound that suppresses the growth of cancer cells bearing a specific mutant gene.
Previous studies have shown that cancer cells with mutant gene p53 appear to be more dependent on DNA2, an enzyme that binds to DNA and plays a role in its replication and repair. DNA2 is overproduced in cancer cells, particularly in those carrying a p53 mutation.
DNA2 overexpression driven by mutant p53 gives cancer cells a survival advantage: It is associated with advanced disease, poor outcomes, and acquired resistance to therapies.
Baylor College of Medicine researchers investigated whether inhibiting the DNA2 function associated with the p53 mutation would stop cancer progression in cell-based and in animal models. They took a computational approach that enabled them to identify the structures that are involved in DNA2 function. Then, they developed compounds that would fit into and bind to those structures, thus preventing DNA2 from binding to DNA and conducting its function.
Researchers identified compound d16, which they tested in cell-based and in animal models, and showed that it can suppress cancer growth when compared to placebo treatment.
The team also discovered that d16 can inhibit a DNA repair process and make cancer cells become sensitive to PARP inhibitors. PARP inhibitors are a class of FDA-approved drugs that only work in certain cancers with mutations in BRCA1 or BRCA2. Now, the combination of d16 with PARP inhibitors can work to treat many cancer cells harboring wild-type BRCA genes. They also found that treating with inhibitor d16 overcame some conventional therapy resistance, making the cells susceptible again to other drugs that were ineffective before.
Researchers are hopeful these new findings will lead to new, more effective drug therapies in people with hard-to-cure cancers.
Researchers at Baylor College of Medicine and collaborating institutions have investigated potential vulnerabilities in Triple-Negative Breast Cancer (TNBC), an aggressive tumor with very poor prognosis and limited therapeutic targets, that could lead to novel therapies and improved outcomes for this devastating condition.
In diverse TNBC animal models, researchers found that targeting the protein eIF4A with the small-molecule drug Zotatifin not only suppresses tumor cell proliferation by robustly inhibiting the production of several tumor-promoting proteins, but also remodels the tumor immune microenvironment into one that favors tumor elimination.
Combining Zotatifin with chemotherapy drug carboplatin, which is typically used as standard treatment for TNBC, produced significantly better results than each treatment alone, as the combination markedly prolonged survival in the animal models.
Another benefit of the small molecule is that it allows for lowering the dose of chemotherapy. Currently, patients are treated with a maximum tolerated dose of chemo, which carries many toxicities and negative side effects. But when chemo is combined with the small inhibitor, its dosage can be cut in half (the minimum effective dose), and achieve good survival benefits and reduce the toxicity.
Targeting the process for synthesizing proteins the cancer needs to grow, is producing promising results. Researchers are hopeful these findings could help inform future clinical trials and positively impact the treatment of TNBC patients.
A team led by researchers at Baylor College of Medicine is coming closer to delivering on the promise of personalized breast cancer therapy with a strategy to predict the most likely response of a cancer to a specific, less toxic treatment regimen.
In a study recently published in the journal Clinical Cancer Research, scientists developed and validated in clinical trials a multiparameter molecular classifier test to predict with a high degree of confidence which patients with HER2-positive (HER2+) breast cancer would be candidates for anti-HER2 therapy alone, without the need for chemotherapy. The molecular classifier also accurately identifies patients whose tumors may need chemo or other targeted therapies.
Historically, HER2+ breast cancer, which represents about one of every five breast cancers, was treated only by chemotherapy, but patient outcomes were poor. This changed in the late 1990s when the introduction of anti-HER2 therapy, drugs that block the growth effects of HER2, transformed the treatment of this disease.
Researchers first observed that blocking HER2 with two different drugs in breast cancer cells growing in culture was superior to one drug, and then confirmed this in mice with HER2+ human tumors. The tumors disappeared quickly in all mice, something they had never seen before.
They then translated these discoveries to patients. Specifically targeting the HER2 protein with two anti-HER2 drugs (lapatinib and trastuzumab) before surgery resulted in a complete response—meaning disappearance of all cancer in the breast—in about 25-30% of cases to the extent that chemotherapy, which typically is part of the treatment, was no longer needed, sparing patients the toxic effects and cost of chemo.
The challenge was to identify those 30% at the time of diagnosis which would permit a therapy de-escalation approach. Once they accomplished that, researchers would then work to develop a strategy that would distinguish the patients who would respond to anti-HER2.
The discovery promises a way of identifying these patients so they can get the proper, personalized treatment for their cancer that avoids unnecessary treatments and preserves the quality of their lives.
With a five-year survival rate of only 8 percent, pancreatic cancer, the third leading cause of cancer-related deaths in the US, has the lowest survival rate of any major cancer. Unlike breast cancer or colon cancer, however, there isn't a reliable screening method for pancreatic cancer—yet.
Researchers at the Dan L Duncan Comprehensive Cancer Center and the Liver and Pancreas Center at Baylor St. Luke's Medical Center are working together to change that with their research into early detection methods, including genetic testing, which updated national guidelines recommend for anyone diagnosed with pancreatic cancer, regardless of age or family history.
Pancreatic cancer can be difficult to diagnose. Symptoms of pancreatic cancer can be vague, and include back pain, trouble eating, and weight loss. Besides inherited mutations, risk factors for pancreatic cancer include smoking, obesity and heavy alcohol consumption.
Up to 20 percent of pancreatic cancers are thought to be inherited. Some patients will be found to harbor a mutation that explains why that person developed pancreatic cancer. The most common mutation is the BRCA mutation, which predisposes carriers to breast, pancreatic, ovarian, and other cancers. PALB2 is another mutation that increases the risk for breast and pancreatic cancer. There is also a targeted medication for BRCA-related pancreatic cancer, another reason why genetic testing is recommended.
One of the most promising components of pancreatic cancer clinical studies is immunotherapy, which relies on the patient's own built-in defense system against cancers. Multiple clinical trials testing novel immunotherapies in all stages of pancreatic cancer studies are underway at Baylor St. Luke's Medical Center.
The most recent clinical trial is for patients with early-stage cancer who are candidates for surgery. It combines chemotherapy with immunotherapy, followed by surgery. The chemotherapy is used to change the tumor to make it more responsive to immunotherapy before the tumor is surgically removed, creating a strong immune response to where the patient gets lasting protection.
With only about 5 percent of patients with pancreatic cancer participating in such studies, improving outcomes will depend on more patients enrolling in clinical trials.
Colorectal surgeons at Baylor College of Medicine are leading several ongoing, cutting-edge research projects that aim to enhance patient care.
Two National Cancer Institute studies being co-led by Baylor colorectal surgeons are trying to determine if circulating tumor DNA (CtDNA), a new blood test that looks at specific tumor cells circulating in the blood, has a role in colorectal cancer treatment.
Currently, cancer staging and the need for chemotherapy is determined by the presence of cancer in the lymph nodes. The CtDNA has the potential to be better than testing lymph nodes to determine the need for further chemotherapy.
Baylor College of Medicine colorectal surgeons are leading these National Cancer Institute trials with about 250 sites. One trial is looking at patients with stage 2 colon cancer who typically do not get chemotherapy. The trial aims to determine whether chemotherapy might benefit patients who test positive for CtDNA. The second trial is looking at patients with stage 3 cancer who usually get chemotherapy. The trial is investigating the possibility of avoiding chemotherapy if the patient tests negative for CtDNA.
Patients with rectal cancer usually need chemotherapy and radiation before surgery but have different degrees of responses to treatment. The degree of response determines the need and type of surgery needed. Researchers are investigating which patients are going to respond and why, so treatment can be tailored for the best response and improving patient outcomes.
Baylor College of Medicine colorectal surgeons are also studying the efficacy of prehabilitation—that is, physical therapy prior to surgery—in colorectal cancer patients needing surgery. Prehabilitation has been shown to improve postoperative outcomes overall. However, patient compliance with physical therapy exercises before surgery is poor for various reasons. In this study, researchers are providing a novel, in-home table game that makes the patient move and exercise before surgery to see if it improves the wellness of the patient after surgery. The study is looking at the impact of not only prehabilitation but interactive gaming as a tool to motivate and increase compliance.
Results of a new clinical trial involving patients with muscle-invasive bladder cancer may alter the way surgical oncologists typically treat the disease.
The procedure, known as an extended lymphadenectomy, is considered a standard of care and is increasingly used, especially for patients with locally advanced bladder cancer who have a higher risk of lymph node metastases. Removing the bladder as well as all of the lymph nodes in the primary area around the bladder is believed to significantly reduce the risk of cancer recurrence within the pelvis.
But researchers at Baylor College of Medicine and the Southwest Oncology Group (SWOG) Cancer Research Network sought to address an important surgical question: Was the extended lymphadenectomy actually benefiting patients and reducing the risk of recurrent disease or mortality?
With support from the National Cancer Institute and the Canadian Cancer Society, researchers launched a clinical trial and enrolled 658 patients—618 of whom were eligible to be randomly assigned to receive either extended or standard lymphadenectomy—and recruited 36 surgeons, who were required to undergo a credentialing process designed specifically for the study. The researchers then examined whether the patients benefited from extended lymphadenectomy to remove even more lymph nodes from a wider area.
The patients involved in the trial were randomly assigned during their surgery, after the surgeon had determined that the disease had not metastasized beyond the pelvis. All of the patients underwent a standard bilateral pelvic lymphadenectomy. However, those randomly assigned to the investigative arm also underwent an extended lymphadenectomy, with nodes removed at least up to the aortic bifurcation.
Compared with those in the control group, patients in the extended lymphadenectomy group had a median of 39 nodes vs 24 nodes removed, but the percentage of nodes found to contain metastatic disease was similar in both groups. The researchers hypothesized that the patients in the extended lymphadenectomy group would experience improved disease-free and overall survival compared with those in the control group. However, no significant statistical differences were observed between the two groups in disease-free survival.
Further, almost half of patients in the extended lymphadenectomy group experienced adverse events within 90 days of surgery, regardless of attribution, compared with 42% of patients in the control group. The researchers also reported that the number of deaths within 90 days of surgery was greater in the extended lymphadenectomy group (19 vs. seven patients).
The researchers noted that a definitive phase III surgical trial of this sort was an ideal fit for the National Cancer Institute’s National Clinical Trials Network (NCTN) and was uniquely suited for such practice-changing trials led by surgical oncologists.
Glioblastoma (GBM) is the most aggressive and lethal form of brain tumor. Yet, the underlying molecular mechanisms driving GBM infiltration are not fully understood.
Now, researchers from Baylor College of Medicine working in animal models have uncovered a novel process by which neurons in locations remote to the primary tumor provoke expression of genes from glioblastoma that subsequently drive tumor infiltration.
Researchers found that “the tumor microenvironment plays an essential role in malignancy, and neurons have emerged as a key component of the tumor microenvironment that promotes tumorigenesis across a host of cancers.
Recent studies on GBM highlight bidirectional signaling between tumors and neurons that propagates a vicious cycle of proliferation, synaptic integration, and brain hyperactivity. However, the identity of neuronal subtypes and tumor subpopulations driving this phenomenon is incompletely understood.
Researchers showed that callosal projection neurons located in the hemisphere contralateral to primary GBM tumors promote progression and widespread infiltration.
The findings suggest that GBMs receive neuronal inputs from a host of brain regions, implying that exposure to a diverse range of neuroactive compounds can potentially influence tumor growth. It’s now clear that tumor-neuron interactions are more widespread than previously thought.
The study demonstrates that subsets of neurons in locations remote to primary GBM promote malignant progression, and also show new mechanisms of glioma progression that are regulated by neuronal activity.
A highly select group of patients diagnosed with mesothelioma cancer in both the thoracic and abdominal cavities (bicavitary mesothelioma) can still achieve a significant extended survival with an aggressive, lung-sparing surgery that is performed in two stages, concludes a recent study at Baylor College of Medicine.
Mesothelioma is a nearly uniformly fatal tumor that most commonly affects the protective lining of the lungs (pleura). It can also affect the lining of the abdomen (peritoneal) in up to one-quarter of all cases. A more rare form affects the protective lining of the heart (pericardial).
Patients with bicavitary mesothelioma can undergo multimodality therapy, including cytoreductive surgery (CRS) and chemotherapy. CRS for thoracic disease includes a lung-sparing operation called an extended pleurectomy/decortication (ePD) or a lung-sacrificing surgery called an extrapleural pneumonectomy (EPP).
The benefit of CRS for bicavitary disease (chest and abdomen) has been poorly understood. So, over the course of seven-plus years (February 2014-August 2021), Baylor College of Medicine researchers evaluated 440 patients with mesothelioma. Fourteen patients (3%) underwent CRS of both chest and abdomen as a planned two-stage operation. Thirteen of the 14 patients underwent chest surgery prior to abdomen surgery.
For the entire cohort, the median overall survival was 33.6 months, with a five-year survival of 20%. Those who underwent ePD, however, had better outcomes compared to EPP, with median overall survivals of 58.2 months versus 13.5 months, respectively.
Results of the study are one reason Baylor College of Medicine, along with many specialty centers treating mesothelioma, has moved away from the once-groundbreaking EPP surgery to the life-extending ePD procedure.
Immunotherapy ahead of surgery leads to better outcomes for pleural mesothelioma patients who have tumors that can be removed with surgery, according to another recent study by Baylor College of Medicine researchers. The study lays the groundwork for neoadjuvant immunotherapy in mesothelioma.
Immune checkpoint inhibitors are drugs that activate the immune system to fight cancer. They have revolutionized the treatment of cancer in general but only recently have been recognized to have some efficacy in mesothelioma.
Recent research on the efficacy of immunotherapy for patients with unresectable mesothelioma showed favorable outcomes, which led Baylor College of Medicine researchers to study this approach in patients with resectable mesothelioma.
Immunotherapy can activate an immune response that will persist after the tumor is resected. If the tumor tries to recur, the body has an existing memory immune response to fight it.
The researchers continue to follow the participants in the study, who received the treatment more than three years ago. To date, they have found that dual agent immunotherapy increased the number of important types of CD8 T cells in the tumor, which serve as the “soldiers” of the immune response that kill and remember the tumor. Moreover, there has been increased overall survival in patients receiving the dual agent immunotherapy.
A faster, far more accurate pre-operative test for thyroid cancer promises to drastically reduce the number of unnecessary thyroid removals, as well as the need for lifelong hormone replacement therapy and other negative consequences that people experience after having their thyroid removed.
About 52,000 new cases of thyroid cancer are diagnosed each year in the U.S. The most common test for it, called fine-needle aspiration (FNA), is inconclusive about 20% of the time. In those cases, the patient may receive a follow-up genetic test that also can produce false-positive results. As a preventive measure, doctors often recommend removing part or all of the thyroid. Yet, thousands of patients who have the surgery every year later learn that they don’t have cancer.
To change those outcomes, researchers at Baylor College of Medicine and The University of Texas at Austin are developing a new metabolic thyroid test that employs mass spectrometry imaging to identify metabolites produced by cancerous cells. Clinical studies have shown that the imaging acts as a kind of diagnostic fingerprint that vastly reduces the number of false-positive diagnoses.
Over a two-year period, the researchers worked on identifying these diagnostic metabolic fingerprints using 178 patient tissues before starting a pilot clinical study. During the clinical study, 68 new patients were tested, nearly a third of whom had received inconclusive FNA results. Only about one in 10 of the new metabolic thyroid tests returned a false positive, which could have prevented 17 patients in the study from undergoing unnecessary surgeries.
Dr. James Suliburk, a co-principal investigator and head of endocrine surgery at Baylor College of Medicine, collected FNA biopsies from the patients and used the technology in a pilot trial to demonstrate the accuracy of the new test.


James W. Suliburk, MD, FACS
Permanent ostomy bags, long hospitalizations, and narcotics-based pain management are quickly becoming things of the past for patients recovering from colorectal cancer surgery. Thanks to Baylor’s multidisciplinary approach to treating this type of cancer, patient outcomes have vastly improved with faster recovery times, a lower risk of mortality, and a lower rate of recurrence.
Restorative colorectal surgeons, for instance, are making the permanent colostomy bag obsolete by employing more specialized techniques than traditional surgery that reconnect the intestines with the anus. Using multiple methods, including robotic transanal total mesorectal excision, allows for better visualization, so surgeons are able to remove the entire tumor while avoiding a permanent ostomy bag. As a result of such approaches, outcomes from rectal cancer surgery are much improved, reducing the patient’s length of stay in the hospital, as well as the chances of death or the tumor coming back, notes Dr. Atif Iqbal, Chief of Colorectal Surgery at Baylor St. Luke’s Medical Center.
The use of enhanced recovery after surgery (ERAS) protocols, which rely on evidence-based medicine, has also significantly reduced the average length of a hospital stay for patients after major abdominal surgery from 7-12 days to between 1-3 days. Previously, patients were told not to eat or drink starting at midnight on the day of surgery, even if their case was later in the day. Because this can cause dehydration and low-sugar levels before they go into the operating room, patients are now told to continue their liquid diet up to three hours before the surgery.
In addition, most patients also are able to avoid tubes in their nose/mouth or drains after surgery and no longer have to wait for their bowels to start functioning before they are able to eat or be discharged from the hospital. Patients can have a liquid diet immediately following their surgery and go to a regular diet the next morning. “We have found that the quicker we feed them, the better the patients do,” Iqbal said.
New approaches to pain management have also eliminated the use of dangerously addictive opiates. Many surgeons are now giving a combination of different non-narcotic medications to provide effective pain control in both the postoperative inpatient and outpatient settings.
Choosing a multidisciplinary center like Baylor can make all the difference in colorectal cancer patient outcomes.
Patients should look for a restorative colorectal surgeon and a hospital where a high volume of similar cases have been treated and where the care is not fragmented. A true multidisciplinary program brings all specialists the patient needs into one location, which provides for better communication and allows the patient’s health care team to work together to explore the best medical or surgical option and achieve the most successful outcome for the patient.

Organ transplant patients who travel great distances for lifesaving care at Baylor St. Luke's Medical Center benefit from the highest level of critical care in Baylor St. Luke’s Lung Transplant Program, which has gained an international reputation for the treatment of advanced lung disease and transplant care.
Led by Dr. Puneet Garcha, Medical Director of Lung Transplantation at Baylor St. Luke’s and Associate Professor of Pulmonary Medicine at Baylor College of Medicine, and Dr. Gabriel Loor, Surgical Director of Lung Transplantation at Baylor St. Luke’s and Associate Professor of Surgery at Baylor College of Medicine, the program serves patients from around the US and other countries who are often denied by other providers because the cost of insurance is prohibitive, the patient has a poor prognosis, or other reasons.
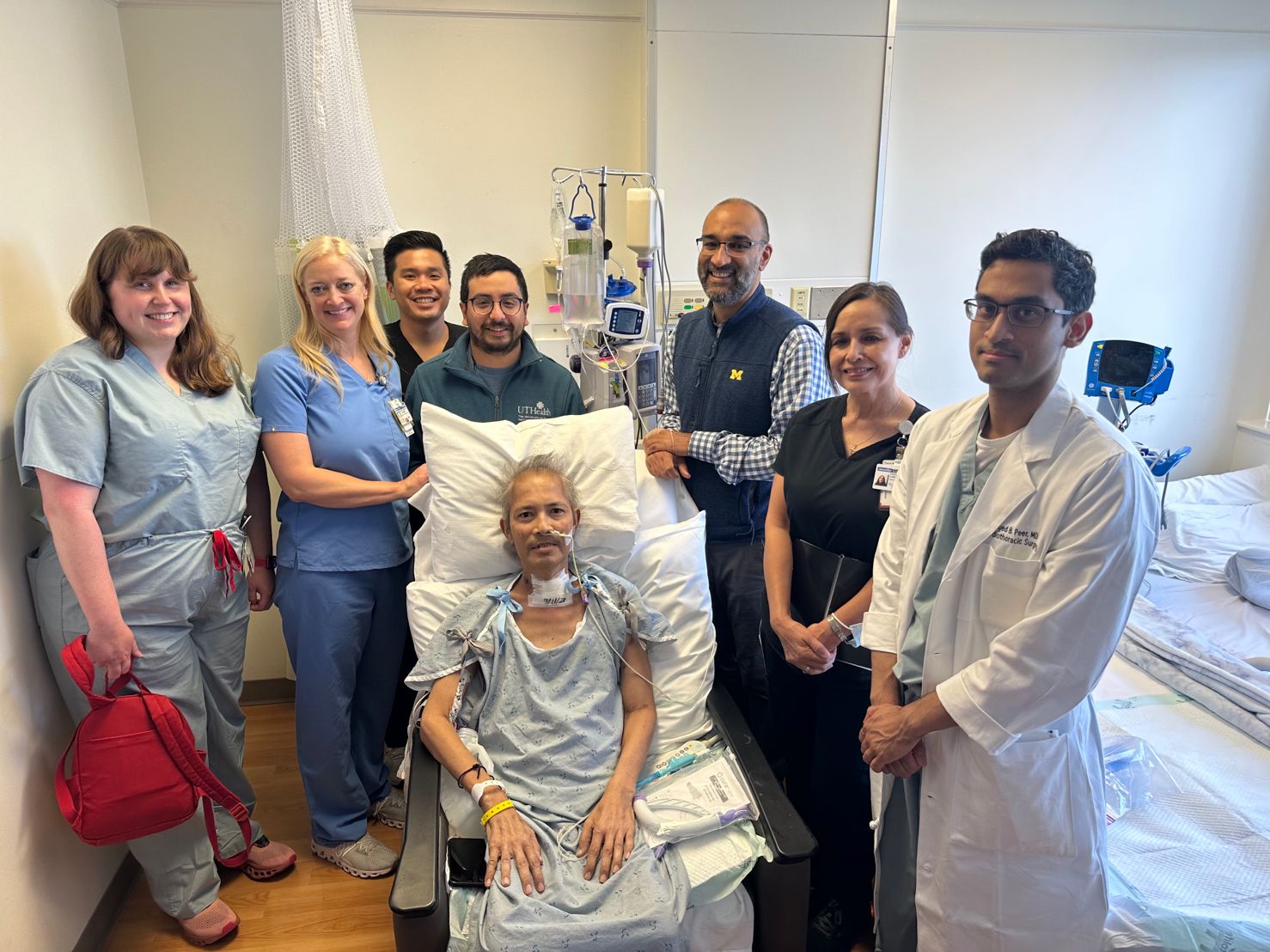
One recent double lung transplant patient is a case in point. A 54-year-old man, a resident of Hawaii, had developed pneumonia, which progressed to respiratory failure that severely damaged both of his lungs. Hospitalized locally, he was intubated and on mechanical ventilation. The patient, who was previously in good health and had no major health issues, also experienced respiratory and kidney failure, infection, gastrointestinal bleeding, and physical deconditioning.
Baylor St. Luke’s accepted the patient after he was rejected by other hospitals. He was airlifted over the summer from Hawaii to Houston, where he spent a month in the care of Baylor’s ICU nursing staff and therapists before being prepped for a bilateral lung transplant.
“We were able to secure a donor lung pretty quickly, and his health improved dramatically soon after the procedure,” said Dr. Loor.
Three months after the double transplant, the patient continued his recuperation in Houston, where he received physical therapy and occupational therapy every week, and re-established living independently. His prognosis is good for a complete recovery, Dr. Loor added. This patient’s experience is just one of many stories that reflect the quality of the critical care at Baylor St. Luke's Lung Transplant Program.

The discovery and application of biomarkers have revolutionized the treatment of patients with lung cancer, heart failure, and myocardial ischemia. Yet it has not yet been applied to the care of patients in whom complications develop after a lung transplant.
Recently, a team of researchers from Baylor College of Medicine and the Texas Heart Institute conducted one of the largest single-center studies of biomarkers in lung transplant patients experiencing primary graft dysfunction (PGD) to discover what biomarkers might aid in earlier detection and improved patient care and outcomes.
PGD is the main cause of chronic illness and death for patients who undergo a lung transplant. There is no cure for PGD. While patients can be successfully treated with supportive care, that alone cannot prevent the potential for irreversible harm to the donor allograft or other end organs.
Early detection of PGD could be improved, however, with the ability to accurately map its molecular signature and specific biomarkers.
The study sought to validate the utility of protein biomarkers for detecting the severity and duration of PGD. The researchers used the most updated PGD grading guidelines, a contemporary cohort of 40 lung transplant recipients, and novel statistical methods to aid in detecting a wide breadth of biomarkers.
Their findings suggest that unique inflammatory protein expression patterns may indicate the severity and duration of PGD. The clinical use and continued examination of these biomarkers may not only help detect PGD early on, but also predict its progression, provide insights for drug development, and establish better treatment benchmarks.
The study was published in September 2022 in Nature.

Understanding the mechanisms that mediate widespread DNA damage in the cancer genome is of great interest to cancer physicians and scientists because it may lead to improved treatments and diagnosis. In this study, a multi-institutional team led by researchers at the Dan L Duncan Comprehensive Cancer Center, in partnership with Baylor College of Medicine, has brought attention to genomic structural variation as a previously unappreciated mechanism involved in altering DNA methylation, a form of gene control, in human cancers.
The researchers brought together data from whole genome sequencing, gene expression, and DNA methylation from more than 1,400 human cancers. They report in the journal Genome Biology that structural variations consistently altered DNA methylation affecting hundreds of genes, overall reducing the global level of DNA methylation across cancers
In this study, Creighton and his colleagues looked at the effect genomic structural variation has on both DNA methylation and gene expression in human cancers. They analyzed data from two different large science consortiums, the Cancer Genome Atlas and the Pancancer Analysis of Whole Genomes. These data include molecular alterations across the entire genome; that is on both protein-coding genes and on their regulatory regions for thousands of cancers. The datasets include the same information from non-cancerous tissues for comparison.
Working with so many patient samples gave more statistical power to the researchers’ analyses and enabled them to find new genes that might be involved in cancer.

As part of our affiliation with Baylor College of Medicine, our urology team takes part in innovative research that we translate into state-of-the-art clinical options for those we serve.
Applying Genomics to Bladder Cancer Treatment
Dr. Seth Paul Lerner, urologist at Baylor St. Luke’s, and his colleagues at Baylor College of Medicine study the genomic underpinnings of diverse characteristics in patients with muscle invasive bladder cancer as part of the Cancer Genome Atlas Research Network.
He and his team found a connection between cancer subtype and outcomes. “We were able to show that mutation signatures, molecular subtypes, load of new cancer-associated molecules, and known clinical and pathological factors have a very clear influence on overall patient survival,” says Dr. Lerner.
Taking these factors into account allows for more personalized and effective treatment for patients. “Of the 11 patients we identified as having a neuronal subtype, all of those evaluable for objective response responded to the treatment (two complete response, six partial response), or 72% overall. This translated to a very high survival probability, which is unprecedented in advanced bladder cancer,” says Lerner.
“Although this is a small group of patients, it is very exciting to see that our basic research can be directly translated to the clinical setting, allowing us to determine which subtype of bladder cancer has a better chance to respond well to a specific treatment.”- Seth Paul Lerner, MD
Targeting Estrogen Receptors to Reduce Bladder Cancer Recurrence
Patients with low/intermediate-risk bladder cancer often experience recurrences. In the second phase of this study, researchers, including Baylor St. Luke’s and Baylor College of Medicine urologist Dr. Guilherme Godoy, sought to reduce the rate of recurrence by prescribing oral tamoxifen.
This prototypic selective estrogen receptor modulator (SERM) was given to prevent carcinogenesis in these patients. Findings revealed that this regimen reduced and even eliminated marker lesions in participants.
Read the full study here.
The Elkins Pancreas Center at the Dan L Duncan Comprehensive Cancer Center specializes in the treatment of pancreatic cancer, pancreatitis, and other pancreatic diseases through traditional surgery, minimally invasive surgery, and clinical trials.
Here, we offer the full range of pancreatobiliary procedures, including the Whipple, to address conditions like chronic pancreatitis and cancer of the pancreas, ampulla of Vater, duodenum, and the distal bile duct. In fact, Baylor St. Luke’s was among the first hospitals in the nation to offer the robotic Whipple procedure.
The Elkins Pancreas Center is recognized as a Pancreatitis Center of Excellence by the National Pancreas Foundation.
For rectal cancer patients who are not candidates for laparoscopy, Baylor St. Luke’s Medical Center offers Transanal Total Mesorectal Excision (taTME), a “bottom-up” minimally invasive robotic surgery. This procedure is an alternative to conventional surgery for patients with lower rectal cancer. The benefits include better visualization for the surgeon, allowing for more aggressive removal while avoiding the anus.